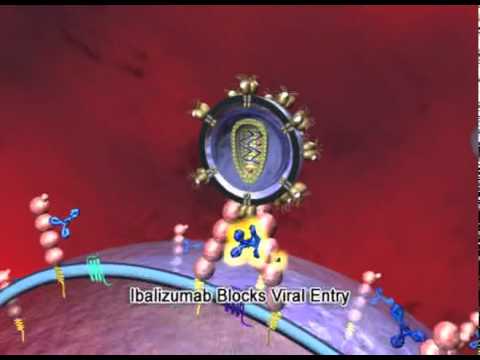
Ibalizumab: First Long Acting HIV Treatment Available Now Via Expanded Access
No one can deny that many patients can now suppress their HIV with effective antiretrovirals (ARVs) that cause fewer side effects. However, a vulnerable and often forgotten minority of people are still struggling with multi-drug resistant HIV (MDR-HIV) while they anxiously wait for access to lifesaving ARVs that would finally control their viral replication. Although …
Ibalizumab: First Long Acting HIV Treatment Available Now Via Expanded Access Read More »