No one can deny that many patients can now suppress their HIV with effective antiretrovirals (ARVs) that cause fewer side effects. However, a vulnerable and often forgotten minority of people are still struggling with multi-drug resistant HIV (MDR-HIV) while they anxiously wait for access to lifesaving ARVs that would finally control their viral replication. Although some of these patients may have developed resistant HIV due to lack of adherence or other issues, many of them have been strictly following their doctors’ orders for years.

They’re often veterans of drug development research who have accumulated HIV resistance as they repeatedly joined ARV studies or traditional expanded access programs of a single new drug out of desperation to control their HIV viral load. As they signed up for studies that helped companies get their drugs approved by the FDA (U.S. Food and Drug Administration), many of these patients were exposed to suboptimal HIV regimens (namely, functional monotherapy or the addition of a single new active ARV to a failing HIV regimen).
Currently, the U.S. Department of Health and Human Services (DHHS) adult HIV treatment guidelines recommend three ARVs be given in combination to suppress HIV. But many patients have HIV that has mutated rendering their virus multi-drug resistant. Those with MDR-HIV cannot construct a viable HIV suppressive regimen with current FDA-approved and commercially available ARVs.
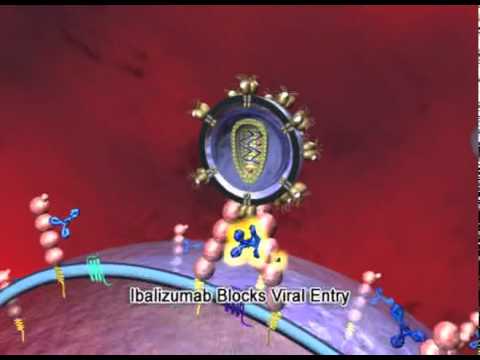
A new HIV medication called Ibalizumab may be approved this year for patients with limited treatment options. It has a completely new mode of action, so most patients should respond to it when using it with at least one other active agent. It is different from the entry inhibitor maraviroc (Selzentry, Celsentri) in that it blocks the CD4 receptor on T cells rather than blocking the CCR5 co-receptor. This means it could be effective against virus that uses either the CCR5 or CXCR4 co-receptor. It is a genetically engineered monoclonal antibody administered once every two weeks intravenously.
In the manufacturer’s website (Taimed Bilogics), the drug is described as : “TMB-355(Ibalizumab) is a humanized monoclonal antibody (mAb) and a member of an emerging class of HIV therapies known as viral-entry inhibitors. This drug candidate is distinct from other entry inhibitors in that it binds to the CD4 molecule, the primary receptor for HIV infection, thereby interfering with the penetration of the virus into the cell. It is the first entry-blocking humanized mAb to treat HIV/AIDS. TMB-355 caught the attention of the scientific community in February 2003, when results from the phase-1, single-dose, intravenous infusion (i.v.) clinical trial showed a transient but clinically significant reduction in the patients’ viral load. Moreover, it was well tolerated with no evidence of adverse effects on CD4 T-cells of treated subjects unlike the majority of approved drugs for HIV. U.S. FDA granted TMB-355 fast track status in October 2003. The phase-2a clinical trial was successfully completed in 2006, with the results showing a clean safety profile and clear antiviral activity (10-fold reduction in viral load). The Phase-2b clinical trial was also successfully completed in 2011, with the confirmation of a good safety profile and strong antiviral activity in HIV patients with multidrug resistance (MDR). U.S. FDA granted orphan drug designation of TMB-355 for HIV patients with multidrug resistance in 2014. Moreover, TaiMed received breakthrough therapy designation from the U.S. FDA for TMB-355 in 2015, which provides the privilege for a rolling biologics license application (BLA) submission. A pivotal phase-3, single-arm clinical trial with patient number of 40 had completed in October, 2016. The clinical trial results, along with other available CMC, pre-clinical, and clinical data, will be submitted as BLA package in 2016 under rolling review. TaiMed Biologics also developed a subcutaneous injection (s.c.) dosage form and the phase-1 human pharmacokinetics bridging study was completed in 2012. Currently, TMB is also developing an intramuscular injection (i.m.) dosage form and a phase-1/2 study for HIV-negative and naive HIV-positive subjects had completed in 2016.” Source
The manufacturer of this biologics product is now providing free access of this treatment option via their expanded program.
Talk to your doctor about this option if you have been told that your virus has resistance to nucleosides, non-nucleosides and protease inhibitors. Remember: This product needs to be used with at least one more active drug to which your virus has not developed resistance. Failure to do so will result in resistance to ibalizumab.
Note from Nelson Vergel: I have been on this product for over 5 years and attribute it to saving my life.
More information:
LONG-ACTING IBALIZUMAB IN PATIENTS WITH MULTI-DRUG RESISTANT HIV-1: A 24-WEEK STUDY