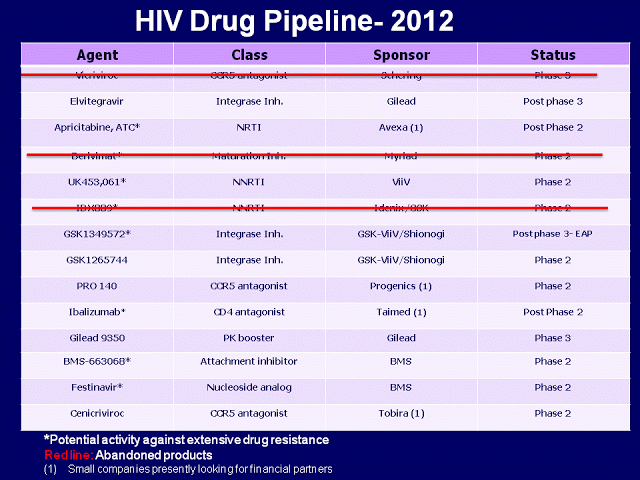
Gilead
has completed and released 48-week data from ongoing phase 3 studies of a
four-drug fixed dose combination (elvitegravir, cobicistat, tenofovir, and FTC-
called QUAD), as it has with studies of the integrase inhibitor elvitegravir
and the pharmacokinetic booster cobicistat.
QUAD will likely be approved for
treatment naive patients after August this year.
has completed and released 48-week data from ongoing phase 3 studies of a
four-drug fixed dose combination (elvitegravir, cobicistat, tenofovir, and FTC-
called QUAD), as it has with studies of the integrase inhibitor elvitegravir
and the pharmacokinetic booster cobicistat.
QUAD will likely be approved for
treatment naive patients after August this year.
Results from phase 3 studies of the integrase
inhibitor dolutegravir (formerly S/GSK-572) have been submitted to the FDA.
This drug may be approved later this year for treatment naive and experienced
patients. This drug is now available via expanded access to patients who have
developed resistance to raltegravir. As with any other drug, it has to be combined with at least one fully active medication and a background therapy for it to control HIV. But some patients do not have another medication to use with dolutegravir, so they will have to wait for other new drugs to be available via expanded access or single patient access programs in the future. Another HIV medication with a new mechanism of action may not be approved in the next 2.5- 3 years.
inhibitor dolutegravir (formerly S/GSK-572) have been submitted to the FDA.
This drug may be approved later this year for treatment naive and experienced
patients. This drug is now available via expanded access to patients who have
developed resistance to raltegravir. As with any other drug, it has to be combined with at least one fully active medication and a background therapy for it to control HIV. But some patients do not have another medication to use with dolutegravir, so they will have to wait for other new drugs to be available via expanded access or single patient access programs in the future. Another HIV medication with a new mechanism of action may not be approved in the next 2.5- 3 years.
Phase 2 studies continue or are expected for
an attachment inhibitor (BMS-663068) that may work for HIV mutidrug resistant
virus, and a CCR5 inhibitor (cenicriviroc, formerly TBR-652) for treatment
naive patients. BMS also has a continued with the developement of Festinavir , a nucleoside reverse transcriptase inhibitor that is active against HIV resistant to both abacavir and tenofovir, making the drug a candidate for people with multi-drug resistant (MDR) strains of the virus.
an attachment inhibitor (BMS-663068) that may work for HIV mutidrug resistant
virus, and a CCR5 inhibitor (cenicriviroc, formerly TBR-652) for treatment
naive patients. BMS also has a continued with the developement of Festinavir , a nucleoside reverse transcriptase inhibitor that is active against HIV resistant to both abacavir and tenofovir, making the drug a candidate for people with multi-drug resistant (MDR) strains of the virus.
Phase
2 results for the attachment inhibitor ibalizumab were presented last September
at the ICAAC conference. Ibalizumab’s manufacturer, Taimed Biologics, is also testing a subcutaneous formulation instead of the intraveneous one used up to now is their studies. Both formulations are dosed every two weeks. As other small biotech companies (Tobira, Avexa, and Progenics), Taimed is currently looking for financial partners to proceed to phase 3 studies.
2 results for the attachment inhibitor ibalizumab were presented last September
at the ICAAC conference. Ibalizumab’s manufacturer, Taimed Biologics, is also testing a subcutaneous formulation instead of the intraveneous one used up to now is their studies. Both formulations are dosed every two weeks. As other small biotech companies (Tobira, Avexa, and Progenics), Taimed is currently looking for financial partners to proceed to phase 3 studies.
Phase 2 results for the NNRTI lersivirine (previously known as UK-453061) in treatment-naive patients were presented at the International AIDS Society’s (IAS) Conference in Rome last summer. GSK-ViiV is exploring this and other drugs as part of a long acting ARV regimen provided via subcutaneous injection (no oral drugs) every week or two weeks. This will revolutionize HIV treatment, in my opinion.
Three
compounds had their development discontinued: vicriviroc (a CCR5
inhibitor), GSK-761 (an NNRTI), and bevirimat (a maturation inhibitor).
compounds had their development discontinued: vicriviroc (a CCR5
inhibitor), GSK-761 (an NNRTI), and bevirimat (a maturation inhibitor).
The
drugs that may work for multidrug resistant HIV are ibalizumab, BMS-663068, dolutegravir, and potentially lersivirine and apricitabine. However, only dolutegravir is now available via expanded access. As a salvage therapy research advocate, I am looking forward to hearing from BMS, Taimed, Progenics about their upcoming phase 3 studies
and future expanded access programs. Stay tuned for an upcoming article in Treatment Issues about salvage therapy access for the dwindling but vulnerable population of patients with extensive HIV multi-drug resistance.
drugs that may work for multidrug resistant HIV are ibalizumab, BMS-663068, dolutegravir, and potentially lersivirine and apricitabine. However, only dolutegravir is now available via expanded access. As a salvage therapy research advocate, I am looking forward to hearing from BMS, Taimed, Progenics about their upcoming phase 3 studies
and future expanded access programs. Stay tuned for an upcoming article in Treatment Issues about salvage therapy access for the dwindling but vulnerable population of patients with extensive HIV multi-drug resistance.
As a side note, some preliminary cure related studies have started enrolling:
HIV Cure Related Studies